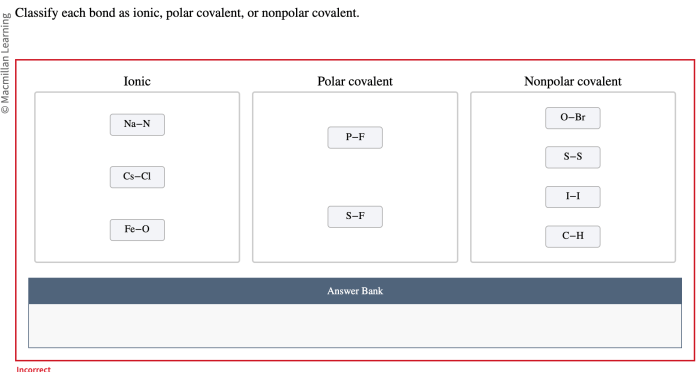
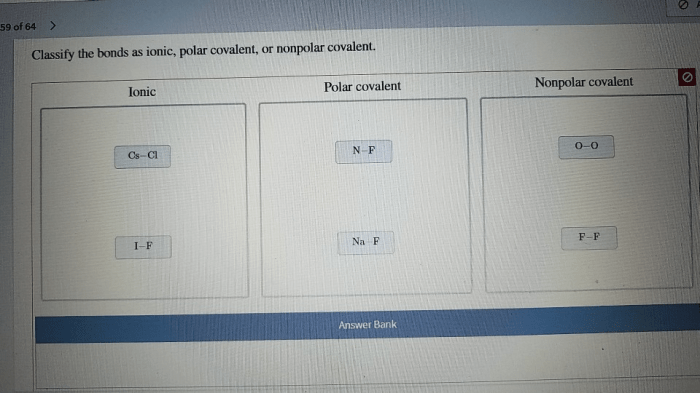
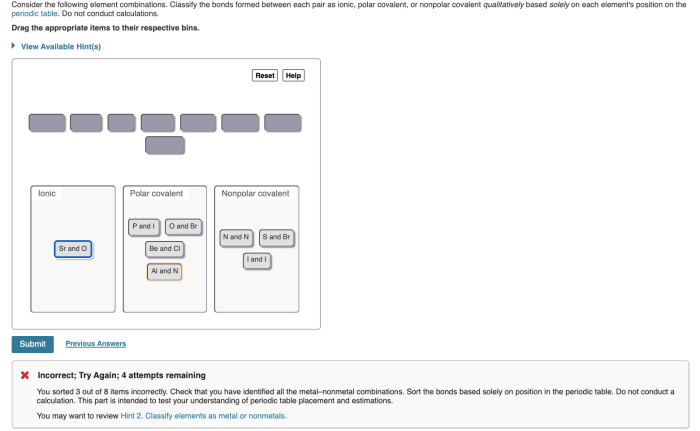
Classify the bonds as ionic polar covalent or nonpolar covalent. The world of chemical bonding is a fascinating one, where atoms interact to form molecules and compounds with unique properties. Understanding the different types of chemical bonds is crucial for comprehending the behavior and reactivity of matter.
In this exploration, we will delve into the realm of ionic, polar covalent, and nonpolar covalent bonds. We will uncover their distinct characteristics, explore how they form, and examine their impact on the properties of various substances.
Ionic Bonds
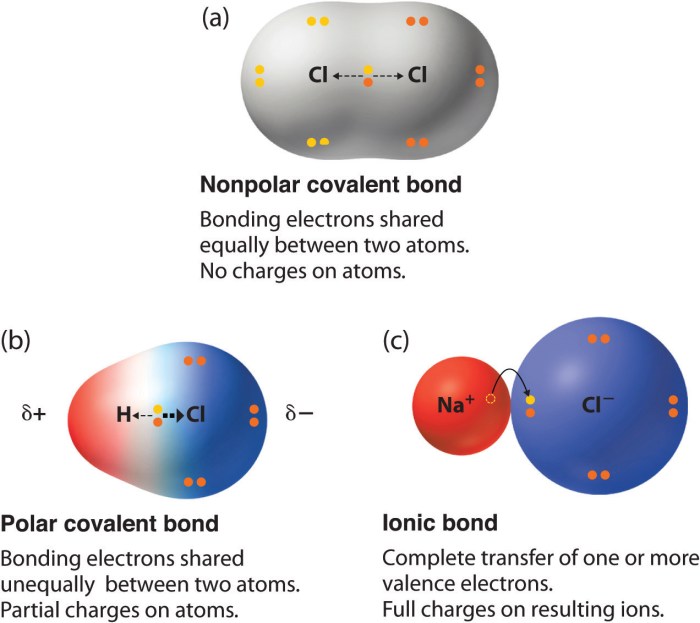
Ionic bonds are chemical bonds formed between atoms with significantly different electronegativities, resulting in the transfer of one or more electrons from one atom to another. The atom that loses electrons becomes a positively charged ion (cation), while the atom that gains electrons becomes a negatively charged ion (anion).
These oppositely charged ions are attracted to each other by electrostatic forces, forming an ionic bond.
Examples of Ionic Bonds, Classify the bonds as ionic polar covalent or nonpolar covalent
- Sodium chloride (NaCl): Sodium (Na) atom loses one electron to chlorine (Cl) atom, forming Na+ and Cl- ions, respectively.
- Potassium fluoride (KF): Potassium (K) atom loses one electron to fluorine (F) atom, forming K+ and F- ions, respectively.
- Calcium oxide (CaO): Calcium (Ca) atom loses two electrons to oxygen (O) atom, forming Ca2+ and O2- ions, respectively.
Properties of Ionic Compounds
| Property | Description |
|---|---|
| High melting and boiling points | Strong electrostatic forces between ions require a lot of energy to overcome. |
| Good electrical conductivity in molten or aqueous solutions | Mobile ions can carry electric current. |
| Poor electrical conductivity in solid state | Ions are fixed in a crystal lattice and cannot move. |
| High solubility in polar solvents | Polar solvents can solvate ions and weaken the electrostatic forces between them. |
Polar Covalent Bonds
Polar covalent bonds are chemical bonds formed between atoms with different electronegativities, but not enough to cause complete electron transfer. Instead, the electrons are shared between the atoms, but one atom has a slightly greater attraction to the electrons than the other.
This creates a partial positive charge on one atom and a partial negative charge on the other, resulting in a polar covalent bond.
Examples of Polar Covalent Bonds
- Hydrogen chloride (HCl): Hydrogen (H) atom has a slightly greater electronegativity than chlorine (Cl) atom, resulting in a partial positive charge on H and a partial negative charge on Cl.
- Water (H2O): Oxygen (O) atom has a greater electronegativity than hydrogen (H) atoms, resulting in a partial positive charge on H atoms and a partial negative charge on O atom.
- Ammonia (NH3): Nitrogen (N) atom has a greater electronegativity than hydrogen (H) atoms, resulting in a partial positive charge on H atoms and a partial negative charge on N atom.
Properties of Polar Covalent Compounds
| Property | Description |
|---|---|
| Lower melting and boiling points than ionic compounds | Weaker electrostatic forces between partially charged atoms require less energy to overcome. |
| Poor electrical conductivity in all states | Electrons are not mobile enough to carry electric current. |
| Variable solubility in polar and nonpolar solvents | Solubility depends on the polarity of the solvent and the compound. |
Nonpolar Covalent Bonds
Nonpolar covalent bonds are chemical bonds formed between atoms with equal electronegativities. The electrons are shared equally between the atoms, resulting in no partial charges or polarity. These bonds are typically formed between atoms of the same element or between atoms of different elements with similar electronegativities.
Examples of Nonpolar Covalent Bonds
- Hydrogen gas (H2): Two hydrogen atoms share their electrons equally, resulting in a nonpolar covalent bond.
- Chlorine gas (Cl2): Two chlorine atoms share their electrons equally, resulting in a nonpolar covalent bond.
- Methane (CH4): Carbon atom shares its electrons equally with four hydrogen atoms, resulting in four nonpolar covalent bonds.
Properties of Nonpolar Covalent Compounds
| Property | Description |
|---|---|
| Very low melting and boiling points | Weak dispersion forces between molecules require very little energy to overcome. |
| Poor electrical conductivity in all states | Electrons are not mobile enough to carry electric current. |
| Insoluble in polar and nonpolar solvents | Nonpolar compounds do not interact strongly with either polar or nonpolar solvents. |
Comparison of Ionic, Polar Covalent, and Nonpolar Covalent Bonds: Classify The Bonds As Ionic Polar Covalent Or Nonpolar Covalent
The following table summarizes the key differences between ionic, polar covalent, and nonpolar covalent bonds:
| Property | Ionic Bonds | Polar Covalent Bonds | Nonpolar Covalent Bonds |
|---|---|---|---|
| Electronegativity difference | Large | Moderate | Equal |
| Electron transfer | Complete | Partial | None |
| Bond polarity | Yes | Yes (partial) | No |
| Melting and boiling points | High | Lower than ionic | Very low |
| Electrical conductivity | Good in molten or aqueous solutions | Poor in all states | Poor in all states |
| Solubility | High in polar solvents | Variable | Insoluble in all solvents |
Frequently Asked Questions
What is the key difference between ionic and covalent bonds?
Ionic bonds involve the transfer of electrons, while covalent bonds involve the sharing of electrons.
How does electronegativity affect the polarity of a covalent bond?
Electronegativity determines the extent to which atoms attract electrons in a covalent bond, influencing the polarity of the bond.
Can a molecule have both polar and nonpolar covalent bonds?
Yes, a molecule can contain both polar and nonpolar covalent bonds, resulting in a net polarity or nonpolarity.