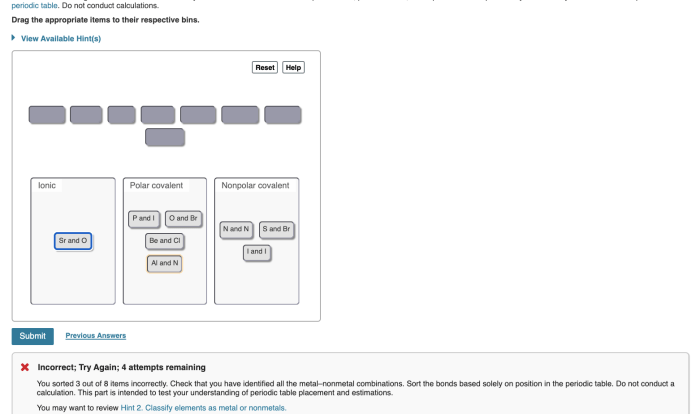
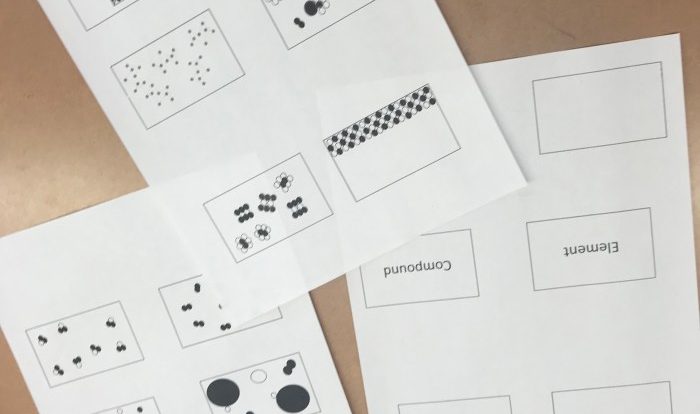
Embarking on an exploration of the fundamental building blocks of matter, this elements compound and mixture worksheet delves into the fascinating world of elements, compounds, and mixtures. Through engaging activities and comprehensive explanations, this resource empowers learners to grasp the intricacies of these chemical entities and their significance in the world around us.
Delving into the properties, classification, and reactions of elements, compounds, and mixtures, this worksheet provides a structured and interactive approach to understanding their unique characteristics and behaviors. By engaging with real-world examples and thought-provoking exercises, learners will develop a deep comprehension of the fundamental principles governing the chemical world.
Introduction to Elements, Compounds, and Mixtures
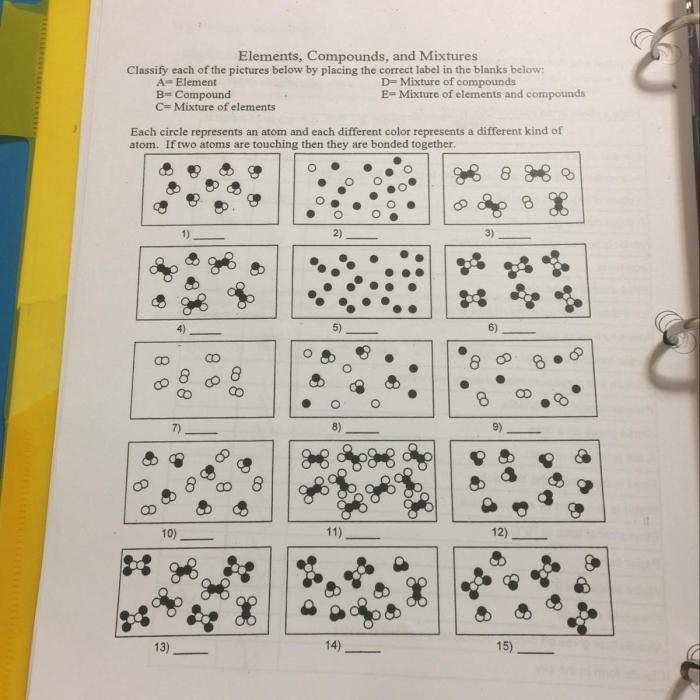
Chemistry is the study of matter and its properties. Matter is anything that has mass and takes up space. It can be classified into three main categories: elements, compounds, and mixtures.
Elements are the simplest form of matter and cannot be broken down into simpler substances by chemical means. They are made up of only one type of atom. Examples of elements include hydrogen, oxygen, and gold.
Compounds are made up of two or more elements that are chemically combined. They can be broken down into their constituent elements by chemical means. Examples of compounds include water (H 2O), salt (NaCl), and sugar (C 12H 22O 11).
Mixtures are made up of two or more elements or compounds that are not chemically combined. They can be separated into their constituent elements or compounds by physical means, such as filtration or distillation. Examples of mixtures include air, seawater, and soil.
Properties of Elements, Compounds, and Mixtures: Elements Compound And Mixture Worksheet
Elements, compounds, and mixtures have different physical and chemical properties. Physical properties are properties that can be observed without changing the chemical composition of the substance. Examples of physical properties include color, density, and melting point.
Chemical properties are properties that describe how a substance reacts with other substances. Examples of chemical properties include flammability, reactivity, and toxicity.
The properties of a compound are different from the properties of its constituent elements. For example, water is a liquid at room temperature, but hydrogen and oxygen are both gases.
The properties of a mixture are typically a combination of the properties of its constituent elements or compounds. For example, air is a gas at room temperature, but it is denser than hydrogen and less dense than oxygen.
Classification of Elements, Compounds, and Mixtures
Elements are classified into three main categories: metals, nonmetals, and metalloids.
- Metals are shiny, malleable, and ductile. They are good conductors of heat and electricity.
- Nonmetals are dull, brittle, and poor conductors of heat and electricity.
- Metalloids have properties of both metals and nonmetals.
Compounds are classified into two main categories: organic compounds and inorganic compounds.
- Organic compounds contain carbon. They are typically found in living things.
- Inorganic compounds do not contain carbon. They are typically found in nonliving things.
Mixtures are classified into two main categories: homogeneous mixtures and heterogeneous mixtures.
- Homogeneous mixtures are mixtures in which the components are evenly distributed throughout the mixture. They appear to be a single phase.
- Heterogeneous mixtures are mixtures in which the components are not evenly distributed throughout the mixture. They appear to be two or more phases.
Chemical Reactions Involving Elements, Compounds, and Mixtures
Chemical reactions are processes in which substances are transformed into new substances. Chemical reactions can occur between elements, compounds, and mixtures.
There are many different types of chemical reactions. Some of the most common types of chemical reactions include:
- Combination reactions
- Decomposition reactions
- Single-replacement reactions
- Double-replacement reactions
- Combustion reactions
Chemical reactions can be used to produce new materials, change the properties of materials, and generate energy.
Applications of Elements, Compounds, and Mixtures
Elements, compounds, and mixtures are used in a wide variety of applications in everyday life.
- Elements are used in the production of metals, plastics, and other materials.
- Compounds are used in the production of drugs, fertilizers, and fuels.
- Mixtures are used in the production of food, beverages, and cosmetics.
The applications of elements, compounds, and mixtures are essential to modern society.
Helpful Answers
What is the difference between an element and a compound?
An element is a pure substance that cannot be broken down into simpler substances by chemical means. A compound, on the other hand, is a pure substance composed of two or more elements chemically combined in fixed proportions.
How are mixtures classified?
Mixtures can be classified as homogeneous or heterogeneous. Homogeneous mixtures have a uniform composition throughout, while heterogeneous mixtures have a non-uniform composition.
What are the different types of chemical reactions involving elements, compounds, and mixtures?
There are many different types of chemical reactions, including combination reactions, decomposition reactions, single-displacement reactions, double-displacement reactions, and combustion reactions.